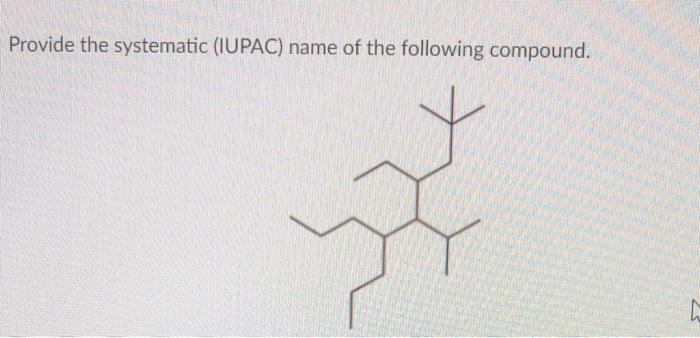
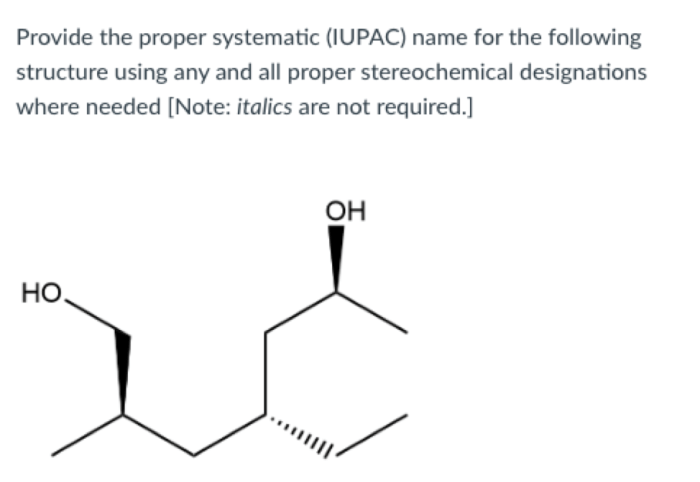
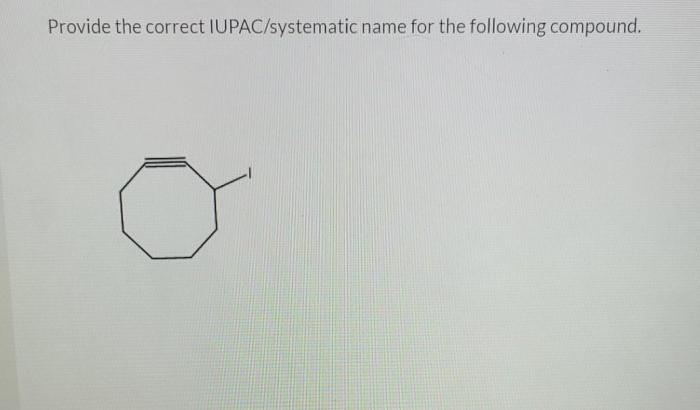
Give the systematic iupac name for the following – In the realm of chemistry, the systematic naming of organic compounds is a fundamental skill that allows scientists to communicate precisely about the structures and properties of these molecules. The International Union of Pure and Applied Chemistry (IUPAC) has established a set of guidelines for naming organic compounds, known as IUPAC nomenclature, which provides a standardized and unambiguous system for identifying and describing these substances.
This comprehensive guide will delve into the intricacies of IUPAC nomenclature, empowering you with the knowledge to decipher and assign systematic names to a wide range of organic compounds. We will explore the fundamental principles, identify functional groups, select parent chains, number carbon chains, name substituents, and navigate special cases to ensure a thorough understanding of this essential aspect of chemical communication.
IUPAC Nomenclature Principles: Give The Systematic Iupac Name For The Following
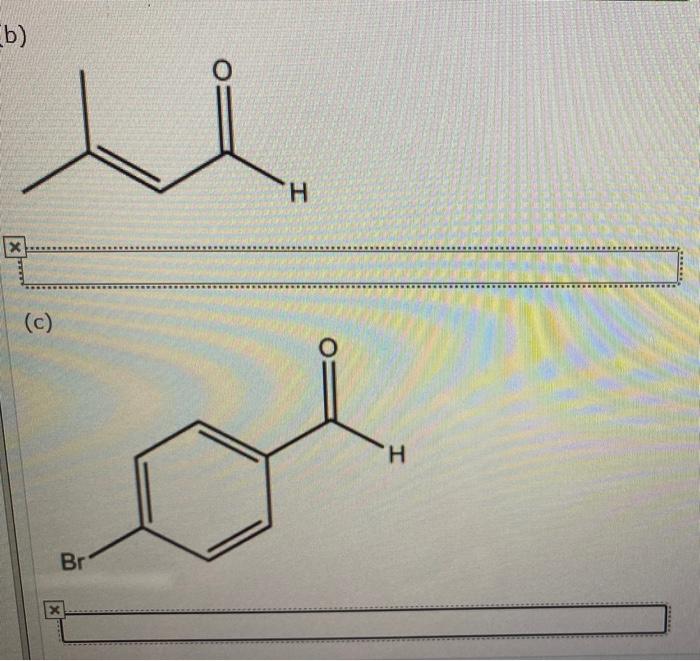
The International Union of Pure and Applied Chemistry (IUPAC) has established a set of rules for naming organic compounds. These rules are designed to ensure that compounds have a unique and systematic name that accurately reflects their structure.
The fundamental principles of IUPAC nomenclature include:
- The name of a compound is based on its parent chain, which is the longest continuous chain of carbon atoms in the molecule.
- The substituents on the parent chain are named and numbered according to their position on the chain.
- The name of the compound is constructed by combining the names of the parent chain and the substituents.
For example, the IUPAC name for the compound CH3CH2CH2CH2CH3 is pentane. The parent chain is pentane, which is a five-carbon chain. The substituents on the parent chain are two methyl groups, which are named according to their position on the chain (1-methyl and 2-methyl).
The name of the compound is constructed by combining the names of the parent chain and the substituents, which gives pentane.
Identifying Functional Groups
Functional groups are atoms or groups of atoms that give organic compounds their characteristic chemical properties. The most common functional groups include:
- Alkanes: Contain only carbon and hydrogen atoms and have the general formula CnH2n+2
- Alkenes: Contain a carbon-carbon double bond and have the general formula CnH2n
- Alkynes: Contain a carbon-carbon triple bond and have the general formula CnH2n-2
- Alcohols: Contain a hydroxyl group (-OH) and have the general formula CnH2n+1OH
- Aldehydes: Contain a carbonyl group (-CHO) and have the general formula CnH2n+1CHO
- Ketones: Contain a carbonyl group (-CO-) and have the general formula CnH2n+1COCH3
- Carboxylic acids: Contain a carboxyl group (-COOH) and have the general formula CnH2n+1COOH
Functional groups are prioritized in IUPAC nomenclature according to their seniority. The seniority of functional groups is determined by the number of bonds between the carbon atom in the functional group and the oxygen atom. The higher the number of bonds, the higher the seniority of the functional group.
Parent Chain Selection
The parent chain in IUPAC nomenclature is the longest continuous chain of carbon atoms in the molecule. The parent chain is used to determine the base name of the compound. The rules for selecting the parent chain are as follows:
- The parent chain must contain the maximum number of carbon atoms.
- The parent chain must contain the maximum number of double bonds.
- The parent chain must contain the maximum number of triple bonds.
- The parent chain must contain the maximum number of functional groups.
For example, the parent chain in the compound CH3CH2CH=CHCH2CH3 is pentene. The parent chain is pentene, which is a five-carbon chain with one double bond. The other possible parent chain is butene, which is a four-carbon chain with one double bond.
However, pentene is the correct parent chain because it contains the maximum number of carbon atoms.
Numbering the Parent Chain, Give the systematic iupac name for the following
The parent chain is numbered from one end to the other in order to give the lowest possible numbers to the substituents. The rules for numbering the parent chain are as follows:
- The carbon atom at one end of the parent chain is numbered 1.
- The carbon atom at the other end of the parent chain is numbered as high as possible.
- If there are multiple substituents on the parent chain, the numbers are assigned in order of their priority.
For example, the parent chain in the compound CH3CH2CH=CHCH2CH3 is numbered from left to right. The carbon atom at the left end of the parent chain is numbered 1, and the carbon atom at the right end of the parent chain is numbered 5. The double bond is located between carbon atoms 2 and 3.
Naming Substituents
The substituents on the parent chain are named according to their structure. The rules for naming substituents are as follows:
- Alkyl substituents are named by replacing the -ane suffix of the alkane with the -yl suffix.
- Alkenyl substituents are named by replacing the -ene suffix of the alkene with the -enyl suffix.
- Alkynyl substituents are named by replacing the -yne suffix of the alkyne with the -ynyl suffix.
- Halogen substituents are named by adding the prefix fluoro-, chloro-, bromo-, or iodo- to the name of the halogen.
- Hydroxyl substituents are named by adding the suffix -ol to the name of the parent chain.
- Carbonyl substituents are named by adding the suffix -al to the name of the parent chain.
- Carboxyl substituents are named by adding the suffix -oic acid to the name of the parent chain.
For example, the substituents on the parent chain in the compound CH3CH2CH=CHCH2CH3 are a methyl group, an ethyl group, and a double bond. The methyl group is named methyl, the ethyl group is named ethyl, and the double bond is named ethene.
Multiple Substituents
When there are multiple substituents on the parent chain, the prefixes di-, tri-, tetra-, etc. are used to indicate the number of substituents. The prefixes are placed in front of the name of the substituent. For example, the compound CH3CH2CH(CH3)CH2CH3 is named 2-methylpentane.
The compound CH3CH2CH(CH3)CH(CH3)CH3 is named 2,3-dimethylpentane.
When there are multiple substituents on the parent chain, the numbers are assigned in order of their priority. The substituents are numbered from one end of the parent chain to the other. The substituent with the lowest number is given the lowest priority.
For example, the compound CH3CH2CH(CH3)CH2CH3 is named 2-methylpentane. The methyl group is given the lowest priority because it is located at the second carbon atom from the left end of the parent chain.
Special Cases
There are a number of special cases in IUPAC nomenclature. These cases include:
- Cyclic compounds: Cyclic compounds are named by adding the prefix cyclo- to the name of the parent chain. For example, the compound C6H12 is named cyclohexane.
- Aromatic compounds: Aromatic compounds are named by adding the suffix -ene to the name of the parent chain. For example, the compound C6H6 is named benzene.
- Polyfunctional compounds: Polyfunctional compounds are compounds that contain two or more functional groups. The functional groups are named in order of their priority. For example, the compound CH3CH2CH(OH)COOH is named 3-hydroxybutanoic acid.
The IUPAC rules for naming organic compounds are complex. However, by following the rules carefully, it is possible to name any organic compound.
Clarifying Questions
What is the purpose of IUPAC nomenclature?
IUPAC nomenclature provides a standardized system for naming organic compounds, ensuring clear and unambiguous communication among scientists.
How do I identify functional groups in organic compounds?
Functional groups are specific arrangements of atoms that impart characteristic properties to organic compounds. They can be identified based on their unique chemical structures and reactivities.
What are the rules for selecting the parent chain in IUPAC nomenclature?
The parent chain is the longest continuous carbon chain in the molecule. It is used as the basis for assigning the root name of the compound.